Cancer Outcomes and Cardiopulmonary Toxicities for Black Patients with Breast Cancer Treated with Proton Therapy
Gurbani Singh , BS, BA¹, Sravya Koduri , MD², Manaahil Rao , MD¹, Meira Kidorf, BS¹, Sarah Ruff , NP¹, Akshar Patel, MD¹,³, Søren M. Bentzen , PhD, DMSc⁴, Elizabeth Nichols , MD¹, Sarah McAvoy , MD¹, Melissa A. L. Vyfhuis , MD, PhD¹
- Department of Radiation Oncology, University of Maryland School of Medicine, Baltimore, MD 21201, United States
- Department of Radiation Oncology, NYU Langone Health, New York, NY 10016, United States
- Department of Radiation Oncology, Chesapeake Oncology-Hematology Associates, Glen Burnie, MD 21061, United States
- Department of Epidemiology and Public Health, Biostatistics and Bioinformatics Division, University of Maryland School of Medicine, Baltimore, MD 21201, United States
Corresponding author: Melissa A. Vyfhuis, MD, PhD, MPTC contributor, Department of Radiation Oncology, University of Maryland, Capital Region Health, 9333 Healthcare Way, Largo, MD 20774, United States ([email protected]).
Abstract
Background: Black women have a 40% higher breast cancer mortality rate than White women and are at a higher risk of acquiring cardiovascular disease. Proton therapy can be used to mitigate cardiac radiation exposure; however, proton therapy remains a scarce resource in the United States. We report on the cardiovascular profiles of patients undergoing proton therapy to determine the potential benefit of the therapy for Black women compared with patients of other races.
Methods: We retrospectively analyzed 599 patients with breast cancer who received proton therapy from June 2016 to December 2021 at the Maryland Proton Treatment Center. A variety of sociodemographic, disease, and treatment variables were analyzed using descriptive statistics.
Results: With a median follow-up of 26months (range ¼ 0.47-90months), Black patients made up 31.6% of the population and presented with higher rates of hypertension (P < .001), cardiopulmonary conditions (P < .001), and a higher median body mass index (P ¼ .015) compared with the other cohort, a trend that persisted at the time of post–proton therapy follow-up. Black women had higher rates of triple-negative disease (P < .001), with subsequent greater receipt of neoadjuvant chemotherapy (P ¼ .039). Pulmonary events were 2.6 times more likely to occur in Black patients than in the non-Black cohort after proton therapy (odds ratio ¼ 2.60, 95% CI ¼ 1.39 to 4.88; P ¼ .003).
Conclusions: Black women presenting for proton therapy had higher baseline risks of cardiovascular co-morbidities combined with more aggressive breast cancer biology and a subsequent 2.6-fold increased risk of pulmonary events after proton therapy. Our findings support the use of advanced radiation techniques as a means of sparing important organs at risk, especially in historically marginalized populations.
Introduction
Racial inequities in female breast cancer mortality rates have persisted since the dissemination of early detection and treatment advances in the 1980s.¹ Despite lower incidence rates, Black women have a 40% higher breast cancer mortality rate than White women, and among women younger than 45 years of age, the rate more than doubles.²,³ Contributing factors to such high mortality rates in Black women include a higher proportion of distant-stage disease at diagnosis and unfavorable and more aggressive tumor characteristics, such as triple-negative disease and inflammatory carcinoma, than women of other races.³ Although genetic factors may explain some of the inequities present in Black women with breast cancer, several studies have reported on additional aspects, such as socioeconomic status and inequitable access to high-quality screening and treatment facilities, that can further exacerbate negative cancer outcomes. ³⁻⁶
Furthermore, Black women tend to have a higher risk of acquiring cardiovascular disease (CVD) than women of other races, developing CVD at a younger age, with an associated higher mortality rate.⁷⁻⁹ Stark differences in mortality also exist between racial cohorts in terms of hypertension, stroke, congestive heart failure, and coronary artery disease, where Black women with coronary artery disease have a 69% increased risk of relative mortality compared with White women. Despite the overall declines in CVD mortality rates over the past several decades, Black women aged 35 to 54 years are experiencing such declines at a much slower rate than women in other racial groups.⁹ Similar to what is described for Black women with breast cancer, several risk factors contribute to the disproportionate number of deaths associated with CVD within the Black community, including high rates of obesity, limited access to primary care clinicians, and other socioeconomic contributors.¹⁰
Radiation therapy (RT) is a vital part of adjuvant treatment in the management of breast cancer, particularly in women with locally advanced or recurrent disease, when the clinical target volume can include the breast and chest wall as well as surrounding nodal areas. Despite the locoregional and distant disease-free survival benefit of RT in patients with advanced breast cancer, there is a valid concern about toxicities to surrounding organs at risk; in particular, cardiovascular toxicity, primarily due to the proximity of the left anterior descending coronary artery (LAD) to the chest wall in cases of left-sided breast irradiation.¹¹⁻¹⁴Rates of major coronary events have been shown to increase linearly, with an average of 7.4% increase per Gray of radiation dose to the heart, in addition to a 16.3% increase in CVD within the first 4 years and a 15.5% increase after 5 to 9 years in patients with breast cancer compared with patients who did not receive RT. Furthermore, these cardiovascular events occurred with greater frequency in women with underlying cardiac risk factors than in those women without such factors.¹⁵
Traditional radiation techniques for locally advanced breast cancer include 3-dimensional conformal RT, which can frequently result in time-consuming, complex plans that can still lead to high doses of radiation to the heart and lungs, often having to sacrifice target coverage to protect these organs at risk.¹⁶ Newer technologies, including intensity-modulated RT or volumetric modulated arc therapy, have shown superior, homogeneous target coverage but frequently at the expense of a higher integral dose of radiation to cardiac structures.¹⁷, ¹⁸ Pencil-beam scanning is the newest form of proton therapy and has been shown to be dosimetrically superior to photon therapy in the treatment of patients with breast cancer, offering excellent target coverage as well as a lower integral dose of radiation to critical surrounding structures, such as the heart, LAD, left ventricle, and lungs.¹⁹⁻²¹ Mitigating radiation dose to cardiac substructures with proton therapy can reduce the predicted risk of cardiac toxicity by up to 2.9% compared with conventional techniques, and this risk attenuation is most likely amplified in women who present with co-morbid conditions that already place them at risk for future cardiovascular events.²¹, ²²
(IBM Corp), and tests of statistical significance were 2 sided, with P less than .05 being statistically significant.
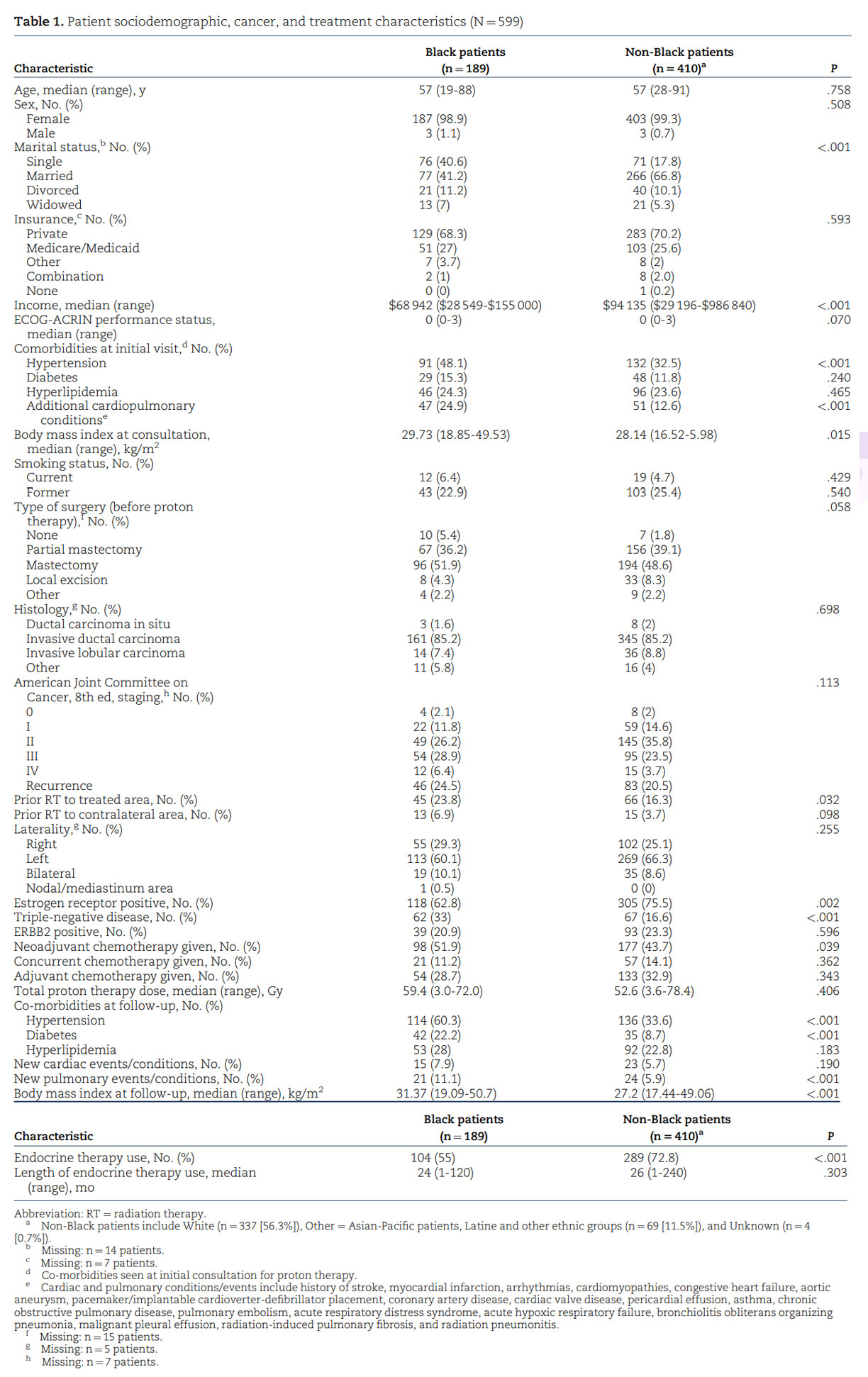
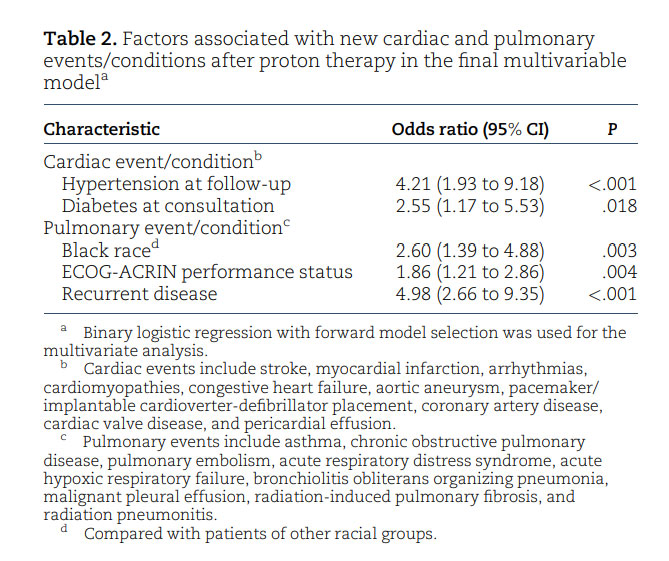
In our analysis, race was not predictive of cancer survival outcomes (Figure 1, A and B), but the volume of the LAD receiving at least 5 Gy and the volume of the left ventricle receiving at least 23 Gy were associated with increased mortality risk after breast cancer radiation with pencil beam scanning proton therapy. Limited data are currently available regarding the relationship between cardiac substructure radiation exposure and the development of short-term and long-term clinical toxicities and survival outcomes for patients, especially when using proton therapy.³⁴, ³⁵ Advances in imaging and ontouring platforms have made it increasingly feasible to delineate the substructures of the heart when creating radiation plans for patients with breast cancer.³⁶⁻³⁸ These advanced plans have demonstrated that dose distribution to the heart is not homogenous, with higher doses often delivered to the apex and apical anterior segments.³⁹ A Swedish study further confirmed these differences, revealing a statistically significant increase in LAD stenosis in patients with left-sided breast cancer who received RT compared with patients with right-sided breast cancer.¹⁴ Thus, mean heart dose alone may be an inadequate dosimetric parameter for assessing RTassociated cardiotoxicity and dose-response relationships. The BreAst Cancer and CArdiotoxicity Induced by RAdioTherapy (BACCARAT) study revealed that more than 55% of patients with left-sided breast cancer receiving mean heart dose less than 3 Gy could still be exposed to LAD doses greater than 40 Gy. Furthermore, the left ventricle and LAD were the most exposed substructures of the heart, with mean doses of 6.2 Gy and 15.7 Gy, respectively.⁴⁰ From a clinical standpoint, left ventricular systolic function plays a vital role in defining the evaluation and management of cardiac disease and in determining patient prognosis.⁴¹ Similar to the predictive value of left ventricular dose in our study, van den Bogaard et al.⁴² concluded that the dose to the left ventricle, particularly a dose of at least 5 Gy, was a better predictor of acute coronary events than mean heart dose or dose to other heart substructures. Associations between LAD dose and increased risk of cardiac events and all-cause mortality in patients with non–small cell lung cancer have also been well established.⁴³ Interestingly, there were differences in the dosimetry seen in certain cardiac substructures between the 2 racial cohorts (Table S1). Our physicians and dosimetry team use standard approaches to contour cardiac substructures, so the difference calculated is most likely attributed to anatomical variations.⁴⁴ Generous treatment of internal mammary nodes in Black women due to more aggressive disease and varying use of inspiratory breath-holding techniques, however, may also have contributed to the differences.⁴⁵
Our study findings provide more evidence of the importance of minimizing dose distribution to individual cardiac substructures, supporting the use of advanced radiation techniques such as deep inspiratory breath holding with photon treatment strategies or proton therapy to spare important organs at risk, such as the heart and lungs, especially in high-risk patients who have existing cardiovascular risk factors.⁴⁶ An increase in the accessibility and implementation of advanced radiation technology in this high-risk subset of patients with breast cancer has the potential to decrease the overall burden of cardiopulmonary toxicity and improve clinical outcomes. Across the country, proton therapy remains a scarce and high-cost resource, especially for minority and marginalized populations residing in low socioeconomic areas.²⁵ A cross-sectional analysis led by the American Cancer Society found that across the 45 proton facilities across the country, Black patients were 33% less likely to be treated with proton therapy than White patients, especially for cancers for which proton therapy is recommended over photon-based RT (odds ratio ¼ 0.67, 95% CI ¼ 0.64 to 0.71). They also found that these disparities increased over time, despite increases in the number of facilities offering proton therapy in the United States (annual percentage change ¼ 0.09, P < .001).²⁵
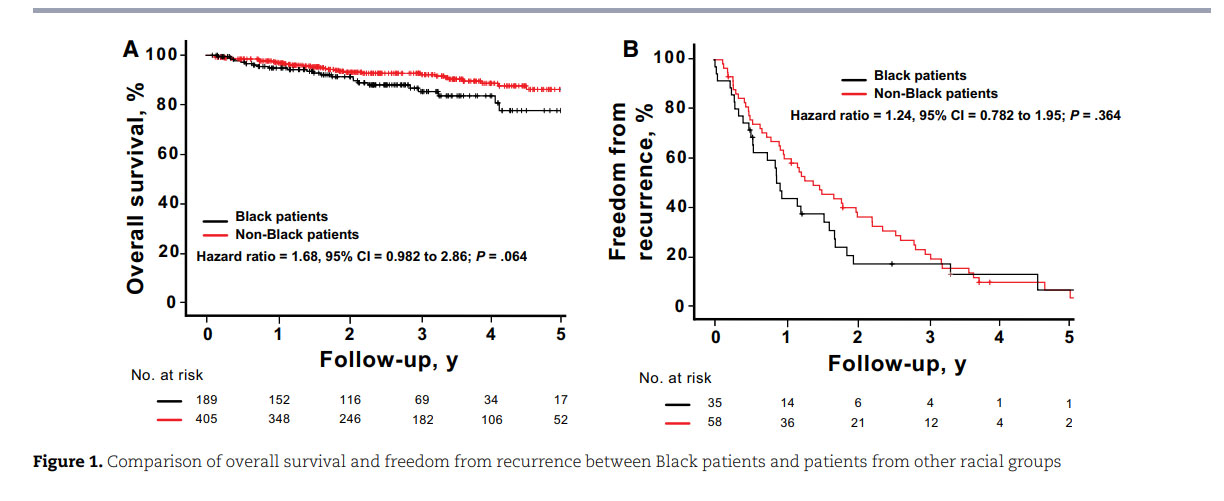
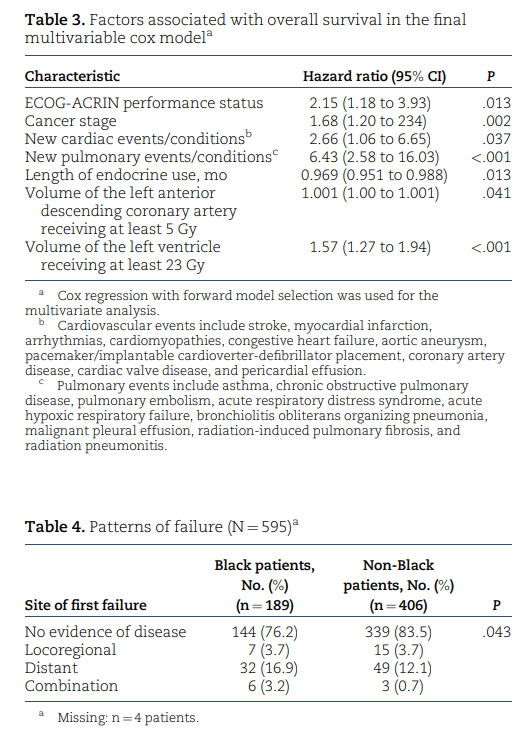
Beyond accessibility to proton therapy, Black women are more likely than women in other racial groups to experience treatment delays and discontinuation as well as longer intervals between abnormal screening mammogram findings and follow-up; they are also less likely to receive guideline-concordant care, even after controlling for insurance status.⁴⁷,⁴⁸ To provide equitable services for Black patients, radiation oncologists and hospital systems must prioritize efforts to mitigate these access barriers. Kronfli et al.⁴⁹ demonstrated that Black patients undergoing RT were more than 3 times more likely than their counterparts in other racial groups to have unmet transportation needs (64% vs 19%; P < .001). Hypofractionated therapy—the delivery of large doses of radiation over fewer courses of treatment—can serve as a potential solution by increasing convenience and minimizing transportation burdens for patients, improving continuity of care and follow-up. Several studies have validated the safety and efficacy in clinical outcomes of various hypofractionated treatment models compared with standard fractionated regimens.⁵⁰,⁵¹ In addition, the coordination of medical appointments among various cancer team members through same-day visits can help minimize transportation needs. The establishment of costneutral payment models to match the cost of proton therapy with that of conventional RT can improve affordability for vulnerable populations who present with increased financial barriers and assistance needs.⁴⁹,⁵² Such collaborative payor environments promote equitable access to advanced radiation treatment modalities for all patients.
This study does have some limitations. First, it was conducted at a single academic institution with a short follow-up period of 26 months. We did not find any significant differences in the development of new cardiac events, overall survival, or freedom from recurrence between the 2 groups; however, this may be attributed to the longer natural history of CVD development and the current lack of sufficient follow-up data. Further study is needed to explore the longer-term development of new cardiac and pulmonary events as well as survival outcomes. Second, nearly one-quarter of our patients underwent previous radiation for a breast malignancy. For the majority of those patients, we did not have access to their previous radiation records and were unable to calculate a total cumulative dose of radiation on important organs at risk, possibly confounding our results. Third, a limited number of patients with stage IV disease were also included in our analysis, and though not considered curable, due to advances in systemic treatment options, patients with limited stage IV breast cancer are living longer and may develop cardiac or pulmonary events, as we tried to characterize.⁵³,⁵⁴ Finally, because of the limitations of a retrospective chart review, we were unable to discern which patients underwent proton therapy due to exceeding organs-at-risk constraints, missing an opportunity to characterize in which dosimetric scenarios proton therapy may be more beneficial in our 2 racial subgroups.
Nevertheless, our study demonstrates that Black patients with breast cancer endure a higher incidence of cardiopulmonary events and high-risk co-morbidities both before and after RT; they also present with more unfavorable disease characteristics. As a result, we theorize that Black patients with breast cancer and a baseline increased CVD risk may derive greater therapeutic benefit from the organ-sparing effects of proton therapy than patients in other racial groups. RadComp will hopefully shed light as to the benefit of proton therapy and its effects on mitigating cardiovascular events, especially within the Black community. Future efforts focused on improving the accessibility of care for Black and other minority populations are imperative to mitigate the ongoing racial inequities in breast cancer outcomes.
Acknowledgments
The authors would like to sincerely thank the MPTC for facilitating this study. The role of the funder is not applicable.
Author contributions
Gurbani Singh, BS, BA (Conceptualization; Data curation; Investigation; Methodology; Writing—original draft; Writing—review & editing), Sravya Koduri, MD (Conceptualization; Data curation; Investigation; Methodology; Writing—review & editing), Manaahil Rao, MD (Conceptualization; Data curation; Investigation; Writing— review & editing), Meira Kidorf, BS (Conceptualization; Data curation; Investigation; Writing—review & editing), Sarah Ruff, NP (Conceptualization; Writing—review & editing), Akshar Patel, MD (Conceptualization; Writing—review & editing), Søren M. Bentzen, PhD DMSc (Conceptualization; Data curation; Formal analysis; Investigation; Writing—review & editing), Elizabeth Nichols, MD (Conceptualization; Data curation; Investigation; Methodology; Writing—review & editing), Sarah McAvoy, MD (Conceptualization; Data curation; Investigation; Methodology; Writing—review & editing), Melissa A. Vyfhuis, MD, PhD (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing— original draft; Writing—review & editing)
Supplementary material
Supplementary material is available at JNCI Cancer Spectrum online.
Funding
None declared.
Conflicts of interest
None declared.
Data availability
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- Jatoi I, Sung H, Jemal A. The emergence of the racial disparity in U.S. breast-cancer mortality. N Engl J Med. 2022;386:2349-2352.
https://doi.org/10.1056/NEJMp2200244 - Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541. https://doi.org/10.3322/caac.21754
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019;69:438-451. https://doi.org/10.3322/caac.2158
- Barber LE, Zirpoli GR, Cozier YC, et al. Neighborhood disadvantage and individual-level life stressors in relation to breast cancer incidence in US Black women. Breast Cancer Res. 2021;23:108. https://doi.org/10.1186/s13058-021-01483-y
- Linnenbringer E, Geronimus AT, Davis KL, Bound J, Ellis L, Gomez SL. Associations between breast cancer subtype and neighborhood socioeconomic and racial composition among Black and White women. Breast Cancer Res Treat. 2020;180: 437-447. https://doi.org/10.1007/s10549-020-05545-1
- Warnecke RB, Campbell RT, Vijayasiri G, Barrett RE, Rauscher GH. Multilevel examination of health disparity: the role of policy implementation in neighborhood context, in patient resources, and in healthcare facilities on later stage of breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2019;28:59-66. https://doi.org/10.1158/1055-9965.EPI-17-0945
- Benjamin EJ, Virani SS, Callaway CW, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67-e492. https://doi.org/10.1161/CIR.0000000000000558. Erratum in: Circulation. 2018;137: e493.
- Kalinowski J, Taylor JY, Spruill TM. Why are young black women at high risk for cardiovascular disease? Circulation. 2019;139: 1003-1004. https://doi.org/10.1161/CIRCULATIONAHA.118.037689
- Williams RA. Cardiovascular disease in African American women: a health care disparities issue. J Natl Med Assoc. 2009; 101:536-540. https://doi.org/10.1016/s0027-9684(15)30938-x
- Balla S, Gomez SE, Rodriguez F. Disparities in cardiovascular care and outcomes for women from racial/ethnic minority backgrounds. Curr Treat Options Cardiovasc Med. 2020;22:75. https://doi.org/10.1007/s11936-020-00869-z
- Darby S, McGale P, Correa C, et al.; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:
1707-1716. https://doi.org/10.1016/S0140-6736(11)61629-2 - Wang K, Tepper JE. Radiation therapy-associated toxicity: etiology, management, and prevention. CA Cancer J Clin. 2021;71: 437-454. https://doi.org/10.3322/caac.21689
- Harris EE, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100-4106. https://doi.org/10.1200/JCO.2005.05.1037
- Nilsson G, Holmberg L, Garmo H, et al. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol. 2012;30:380-386. https://doi.org/10.1200/jco.2011.34.5900
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987-998. https://doi.org/10.1056/NEJMoa1209825
- Salim N, Popodko A, Tumanova K, Stolbovoy A, Lagkueva I, Ragimov V. Cardiac dose in the treatment of synchronous bilateral breast cancer patients between three different radiotherapy techniques (VMAT, IMRT, and 3D CRT). Discov Oncol. 2023; 14:29. https://doi.org/10.1007/s12672-023-00636-z
- Zhao H, He M, Cheng G, et al. A comparative dosimetric study of left sided breast cancer after breast-conserving surgery treated with VMAT and IMRT. Radiat Oncol. 2015;10:231. https://doi.org/10.1186/s13014-015-0531-4
- Chang JS, Chang JH, Kim N, Kim YB, Shin KH, Kim K. Intensity modulated radiotherapy and volumetric modulated arc therapy in the treatment of breast cancer: an updated review. J Breast Cancer. 2022;25:349-365. https://doi.org/10.4048/jbc.2022.25.e37
- Hernandez M, Zhang R, Sanders M, Newhauser W. A treatment planning comparison of volumetric modulated arc therapy and proton therapy for a sample of breast cancer patients treated with post-mastectomy radiotherapy. J Proton Ther. 2015;1:119. https://doi.org/10.14319/jpt.11.9
- Jimenez RB, Hickey S, DePauw N, et al. Phase II Study of proton beam radiation therapy for patients with breast cancer requiring regional nodal irradiation. J Clin Oncol. 2019;37:2778-2785. https://doi.org/10.1200/JCO.18.02366
- Stick LB, Yu J, Maraldo MV, et al. Joint estimation of cardiac toxicity and recurrence risks after comprehensive nodal photon versus proton therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2017;97:754-761. https://doi.org/10.1016/j.ijrobp.2016.12.008
- Kammerer E, Le Guevelou J, Chaikh A, et al. Proton therapy for locally advanced breast cancer: a systematic review of the literature. Cancer Treat Rev 2018;63:19-27. https://doi.org/10.1016/j.ctrv.2017.11.006
- McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31:63-75. https://doi.org/10.1007/s10557-016-6711-0
- Gerodias FR Jr, Tan MK, De Guzman A, et al. Anthracyclineinduced cardiotoxicity in breast cancer patients: a five-year retrospective study in 10 centers. Cardiol Res. 2022;13:380-392. https://doi.org/10.14740/cr1442
- Nogueira LM, Sineshaw HM, Jemal A, Pollack CE, Efstathiou JA, Yabroff KR. Association of race with receipt of proton beam therapy for patients with newly diagnosed cancer in the US, 2004-2018. JAMA Netw Open. 2022;5:e228970. https://doi.org/10.1001/jamanetworkopen.2022.8970
- Bekelman JE, Lu H, Pugh S, et al.; RadComp (Radiotherapy Comparative Effectiveness Consortium). Pragmatic randomised clinical trial of proton versus photon therapy for patients with non-metastatic breast cancer: The Radiotherapy Comparative Effectiveness (RadComp) Consortium trial protocol. BMJ Open. 2019;9:e025556. https://doi.org/10.1136/bmjopen-2018-025556
- Riggan KA, Rousseau A, Halyard M, et al. “There’s not enough studies”: Views of black breast and ovarian cancer patients on research participation. Cancer Med. 2023;12:8767-8776. https://doi.org/10.1002/cam4.5622
- Awidi M, Al Hadidi S. Participation of Black Americans in cancer clinical trials: current challenges and proposed solutions. J Clin Oncol Oncol Pract. 2021;17:265-271. https://doi.org/10.1200/OP.21.00001
- Wang S, Prizment A, Thyagarajan B, Blaes A. Cancer treatmentinduced accelerated aging in cancer survivors: biology and assessment. Cancers (Basel). 2021;13:427. https://doi.org/10.3390/cancers13030427
- Cohen JB, Geara AS, Hogan JJ, Townsend RR. Hypertension in cancer patients and survivors: epidemiology, diagnosis, and management. J Am Coll Cardiol CardioOnc. 2019;1:238-251.
- Xiao Y, Wang H, Tang Y, et al. Increased risk of diabetes in cancer survivors: Aa pooled analysis of 13 population-based cohort studies. ESMO Open. 2021;6:100218.
- Connor AE, Schmaltz CL, Jackson-Thompson J, Visvanathan K. Comorbidities and the risk of cardiovascular disease mortality among racially diverse patients with breast cancer. Cancer. 2021;127:2614-2622. https://doi.org/10.1002/cncr.33530
- Bandera EV, Alfano CM, Qin B, Kang DW, Friel CP, DieliConwright CM. Harnessing nutrition and physical activity for breast cancer prevention and control to reduce racial/ethnic cancer health disparities. Am Soc Clin Oncol Educ Book. 2021;41: 1-17. https://doi.org/10.1200/EDBK_321315
- Ahmadsei M, Thaler K, Gasser E, et al. Dosimetric analysis of 17 cardiac Sub-structures, Toxicity, and survival in ultra central lung tumor patients treated with SBRT. Clin Transl Radiat Oncol. 2023;43:100675. https://doi.org/10.1016/j.ctro.2023.100675
- Hogan JS, Kalaghchi B, Agabalogun T, et al. Effect of dose to the heart and cardiac substructures on cardiac toxicity after breast cancer radiation. Int J Rad OncologyBiologyPhysics. 2023;.117:e180. https://doi.org/10.1016/j.ijrobp.2023.06.1031.
- Tang S, Otton J, Holloway L, et al. Quantification of cardiac subvolume dosimetry using a 17 segment model of the left ventricle in breast cancer patients receiving tangential beam radiotherapy. Radiother Oncol. 2019;132:257-265. https://doi.org/10.1016/j.radonc.2018.09.021
- Yu XL, Zhang Q, Chen JY, et al. Delineation of the cardiac substructures based on PET-CT and contrast-enhanced CT in patients with left breast cancer treated with postoperative radiotherapy. Technol Cancer Res Treat. 2013;12:99-107. https://doi.org/10.7785/tcrt.2012.500299
- Fan LL, Luo YK, Xu JH, He L, Wang J, Du XB. A dosimetry study precisely outlining the heart substructure of left breast cancer patients using intensity-modulated radiation therapy. J Appl Clin Med Phys. 2014;15:4624. https://doi.org/10.1120/jacmp.v15i5.4624
- Moignier A, Broggio D, Derreumaux S, et al. Dependence of coronary 3-dimensional dose maps on coronary topologies and beam set in breast radiation therapy: a study based on CT angiographies. Int J Radiat Oncol Biol Phys. 2014;89:182-190. https://doi.org/10.1016/j.ijrobp.2014.01.055 PMID: 24725701.
- Jacob S, Camilleri J, Derreumaux S, et al. Is mean heart dose a relevant surrogate parameter of left ventricle and coronary arteries exposure during breast cancer radiotherapy: a dosimetric evaluation based on individually-determined radiation dose (BACCARAT study). Radiat Oncol. 2019;14:29. https://doi.org/10.1186/s13014-019-1234-z
- Luis SA, Chan J, Pellikka PA. Echocardiographic assessment of left ventricular systolic function: an overview of contemporary techniques, including speckle-tracking echocardiography. Mayo Clin Proc. 2019;94:125-138. https://doi.org/10.1016/j.mayocp.2018.07.017
- van den Bogaard VA, Ta BD, van der Schaaf A, et al. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol. 2017;35:1171-1178. https://doi.org/10.1200/JCO.2016.69.8480
- Atkins KM, Chaunzwa TL, Lamba N, et al. Association of left anterior descending coronary artery radiation dose with major adverse cardiac events and mortality in patients with non–small cell lung cancer. JAMA Oncol. 2021;7:206-219. https://doi.org/10.1001/jamaoncol.2020.6332
- Duane F, Aznar MC, Bartlett F, et al. A cardiac contouring atlas for radiotherapy. Radiother Oncol. 2017;122:416-422. https://doi.org/10.1016/j.radonc.2017.01.008
- Chapman CH, Jagsi R, Griffith KA, et al. Mediators of racial disparities in heart dose among whole breast radiotherapy patients. J Natl Cancer Inst. 2022;114:1646-1655. https://doi.org/10.1093/jnci/djac120
- Speleers B, Schoepen M, Belosi F, et al. Effects of deep inspiration breath hold on prone photon or proton irradiation of breast and regional lymph nodes. Sci Rep 2023;13:13749. https://doi.org/10.1038/s41598-021-85401-4. Erratum in: Sci Rep. 2023;13:13749. https://doi.org/10.1038/s41598-023-40643-2
- Freedman RA, Virgo KS, He Y, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117:180-189. https://doi.org/10.1002/cncr.25542
- Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2016;2:322-329. https://doi.org/10.1001/jamaoncol.2015.3856
- Kronfli D, Savla B, Lievers A, et al. Identifying psychosocial needs of patients with cancer undergoing curative radiation therapy in an inner-city academic center to address racial disparities. Int J Radiat Oncol Biol Phys. 2022;114:185-194. https://doi.org/10.1016/j.ijrobp.2022.04.003
- Haviland JS, Owen JR, Dewar JA, et al.; START Trialists’ Group. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086-1094. https://doi.org/10.1016/S1470-2045(13)70386-3
- Murray Brunt A, Haviland JS, Wheatley DA, et al.;FAST-Forward Trial Management Group Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395:1613-1626. https://doi.org/10.1016/S0140-6736(20)30932-6
- Mohindra P, Scull A, Molitoris JK, et al. Insurance approval for proton therapy in patients with thoracic malignancies: an experience from a cost-neutral payor environment. Int J Rad OncologyBiologyPhysics. 2021;111:e353-e354. https://doi.org/10.1016/j.ijrobp.2021.07.1058.
- Caswell-Jin JL, Plevritis SK, Tian L, et al. Change in survival in metastatic breast cancer with treatment advances: metaanalysis and systematic review. JNCI Cancer Spectr. 2018;2: pky062. https://doi.org/10.1093/jncics/pky062
- Guo S, Wong S. Cardiovascular toxicities from systemic breast cancer therapy. Front Oncol. 2014;4:4:346. https://doi.org/10.3389/fonc.2014.00346
Connect With MPTC
If you’re a physician or medical professional interested in connecting with MPTC, please complete the form below and we’ll be in touch.


